Gilead Hiv Drugs
Gilead Sciences Inc GILDO on Thursday said its first-quarter product sales rose 16 including 146 billion in sales of its COVID-19 antiviral drug remdesivir but sales of flagship HIV and. Gilead said the COVID-19 pandemic continued to hurt sales of its treatments for hepatitis C and HIV due to fewer people going to their doctors during the pandemic.
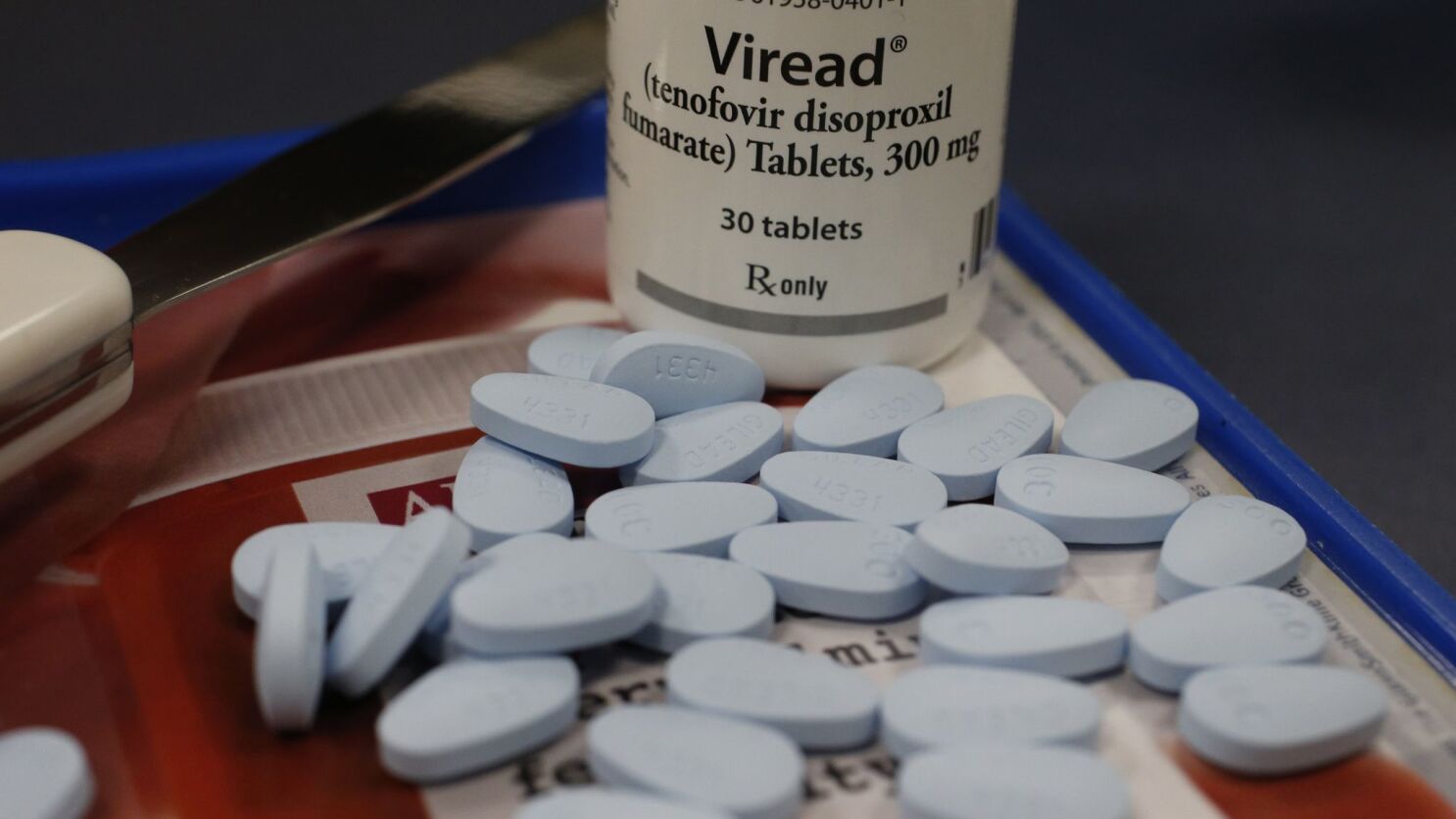
Lawsuits will allege that Gilead Sciences withheld releasing a safer variation of TDF tenofovir alafenamide fumarate TAF.

Gilead hiv drugs. TDF Drugs by Gilead TDF HIV Medications at the Center of Litigation. Gilead is a member of the NASDAQ Biotechnology Index and the SP 500. HHS alleges that its patents have been infringed by Gilead through two drugs indicated for treating HIV-1 infection as well as PrEP.
Truvada Viread Atripla Complera Stribild. But thousands of people living with HIV have allegedly complained of kidney and bone injuries after taking TDF drugs. Truvada has saved countless lives around the world.
Gilead like most other drug companies has been under constant pressure about the high. Gilead Sciences the biopharmaceutical giant sent out a mass mailing in mid-April to people on its antiretroviral drugs Truvada and Descovy. Plaintiffs have alleged that Gilead concealed TDF drug risks and.
Sales of HIV treatments declined 12 to 37 billion as hepatitis C drug revenue fell 30 to 510 million. Gilead Sciences is the leading manufacturer of HIV drugs worldwide. The return address on the envelopes read HIV Prevention Team.
They manufacture tenofovir disoproxil fumarate TDF antiretroviral drugsViread Atripla Complera Stribild and Truvadaused to treat HIV. The drugs are used to reduce the risk of HIV infection a prevention measure called pre-exposure prophylaxis PrEP. Cancer drugs Yescarta and Tecartus generated 160 million and 31 million respectively.
Gilead researchers have developed 11 commercially available HIV medications and are advancing a robust pipeline of next-generation therapeutic options. ˈɡɪliəd is an American biopharmaceutical company headquartered in Foster City California that focuses on researching and developing antiviral drugs used in the treatment of HIV hepatitis B hepatitis C and influenza including Harvoni and Sovaldi. Gilead is committed to advancing care for patients around the world by bringing forward medicines in areas of unmet medical need.
The Gilead HIV drug Bone Injuries include OsteopeniaLoss of bone density with associated harm such as bone fracture or tooth loss Osteoporosis and Osteomalacia. The most widely used AIDS drug is Gileads Truvada 5 which is used for both the treatment of infection and PrEP 6. The two personal injury lawsuits accuse Gilead of intentionally continuing to promote the HIV medication tenofovir disoproxil fumarate TDF which was known to cause serious kidney and bone.
Trodelvy which Gilead recently acquired brought in 72 million. We do this through internal research and development as well as through collaborations with academic and industry partners. PrEP is already a big market for HIV drugs like its blockbuster brands Descovy emtricitabinetenofovir alafenamide and Truvada emtricitabinetenofovir disoproxil fumarate which accounted for.
If the combination were to come to market it would add to Gileads already robust portfolio of HIV drugs which includes Biktarvy the worlds top-selling HIV medication. Truvada emtricitabine200 mgtenofovir alafenamide 300 mg. For more than a decade Gilead Sciences has been a leader in the development of antiretroviral therapy for HIVAIDS.
Tenofovir disoproxil fumarate more commonly known as TDF was approved by the FDA in October 2001 for. Recognizing that the greatest need for HIV treatment is in the least-developed. Gilead reported adjusted earnings of 208 per share edging out the average analyst estimate of 207 as compiled by Refinitiv.
Reuters -Gilead Sciences Inc on Thursday reported first-quarter revenue that fell short of Wall Street estimates as the coronavirus pandemic hurt sales of its flagship HIV and hepatitis C drugs partially offset by sales of COVID-19 antiviral treatment remdesivir. People living with HIV who took tenofovir disoproxil fumarate TDF drugsViread Atripla Complera Stribild and Truvadahave been diagnosed by a physician or other healthcare provider with kidney and bone injuries.
 Supply Of Our Hiv And Hepatitis Meds Is Secure Says Gilead Poz
Supply Of Our Hiv And Hepatitis Meds Is Secure Says Gilead Poz
 Untangling The Trump Administration S Lawsuit Over An Hiv Prevention Drug Science Aaas
Untangling The Trump Administration S Lawsuit Over An Hiv Prevention Drug Science Aaas
 Who Owns H I V Prevention Drugs The Taxpayers U S Says The New York Times
Who Owns H I V Prevention Drugs The Taxpayers U S Says The New York Times
 House Dems Question Gilead S Hiv Drug Donations
House Dems Question Gilead S Hiv Drug Donations
 F D A Approves New H I V Prevention Drug But Not For Everyone The New York Times
F D A Approves New H I V Prevention Drug But Not For Everyone The New York Times
 Gilead 2019 Still Gilead Pharmalive
Gilead 2019 Still Gilead Pharmalive
Gilead Bags Eu Approval For Next Generation Hiv Drug Pmlive
 Generic Hiv Prevention Drug Coming In 2020 Gilead Says
Generic Hiv Prevention Drug Coming In 2020 Gilead Says
Gilead Launches Combination Hiv Pill In Uk And Germany Pmlive
 How Much Is Prep 21k Gilead Drug Blamed In Hiv Prevention Stall Bloomberg
How Much Is Prep 21k Gilead Drug Blamed In Hiv Prevention Stall Bloomberg
 Gilead 2018 The Party S Over Pharmalive
Gilead 2018 The Party S Over Pharmalive
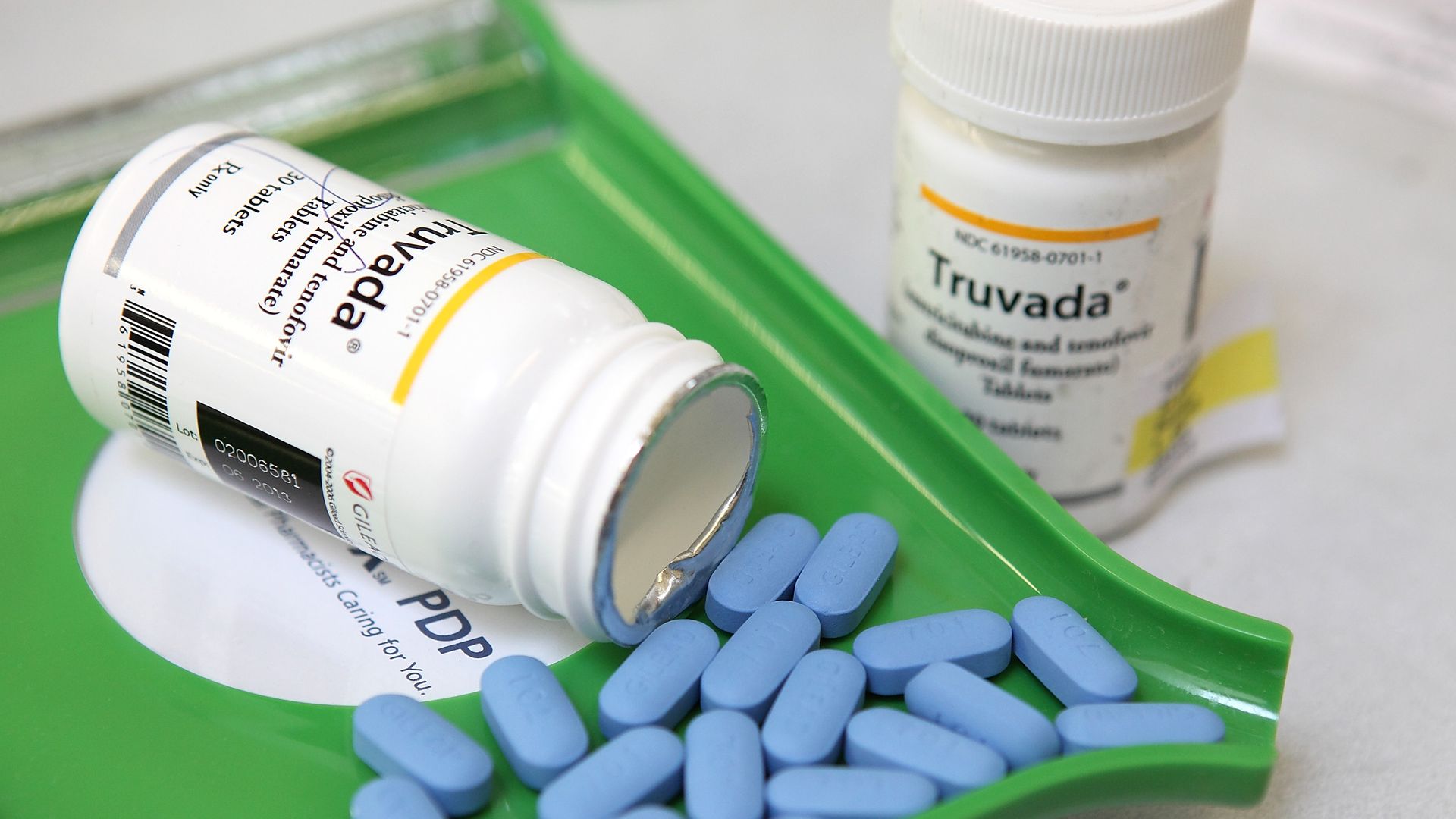 Trump Administration Sues Gilead Over Hiv Drugs Axios
Trump Administration Sues Gilead Over Hiv Drugs Axios
 U K Marketing Police Slam Gilead For Claiming Biktarvy Tolerability Beats Gsk Rival Fiercepharma
U K Marketing Police Slam Gilead For Claiming Biktarvy Tolerability Beats Gsk Rival Fiercepharma
 Gilead Will Donate Truvada To U S For H I V Prevention The New York Times
Gilead Will Donate Truvada To U S For H I V Prevention The New York Times
Comments
Post a Comment